SynFire® iNs are generated using a patented procedure for direct reprogramming and exhibit the main characteristics of human primary neurons, such as expression of typical pan-neuronal markers and complex electrophysiology, including spontaneous and evoked action potentials and synchronized network activity. Neuronal subtype identities have been confirmed by staining and patch clamping.
Pure populations of human neural cell types NeuCyte uses in our culture include:
-
Glutamatergic excitatory neurons
-
GABAergic inhibitory neurons
-
Astroglia

The
SynFire
Advantage
Real human biology
SynFire iN closely resemble real human biology, resulting in better ability to predict responses to compounds
Rapid maturation
Produced through a direct reprogramming approach leading to rapid and homogeneous maturation, SynFire iNs exhibit mature synaptic network activity, such as synchronous bursting phenotypes resembling those in rodent primary cultures appearing within three to four weeks
Reliable, robust and ready-to-use
This reprogramming approach also results in lot-to-lot consistency, providing reproducible results
Flexible modular system
The user can control subtype to subtype relative seeding density and ratio to fit individual projects, allowing for tracking, analyzing and manipulating specific cell types
View the following characterization data of the SynFire iNs:
Expression of pan-neuronal and subtype-specific markers, rapidly mature to form complex networks and cellular morphologies
Pan-Neuron and Subtype Specific Markers
E1
E2

TUJ-1 / GABA / Hoechst
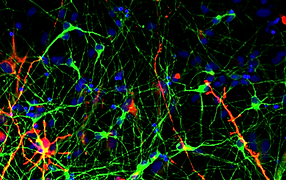
TUJ-1 / GFAP / Hoechst
Elaborate Networks
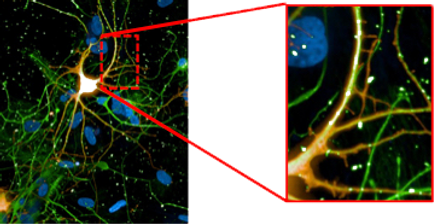
tdTomato / TUJ-1 / DAPI
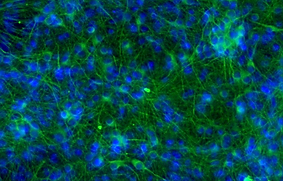
TUJ-1 / DAPI

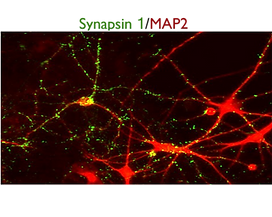
Synapsin 1 / MAP2
Pan-Neuron and Subtype-Specific Markers
A
C
%20data.png)
B
D
Elaborate Networks
E
F

G

tdTomato/TUJ-1/ DAPI
SynFire iNs exhibit mature neuronal characteristics through immuno-staining. The modular aspect of SynFire neural cells allow for defined co-culture conditions and specific ratios of mixed neuronal subtypes, including inhibitory GABAergic neurons. SynFire iNs show expression of neuronal markers within 3 days, formation of spontaneous action potentials within 6-8 days and synchronized neuronal network activity within 21-28 days.
(A) Pan-neuronal marker MAP2 / Astroglia marker GFAP / Nuclear staining DAPI. (B) Pan-neuronal marker β3-Tub (TUJ1) / Inhibitory neuron GABA-A receptor, α1 / Nuclear staining DAPI. (C) Pan-neuronal marker MAP2 / Synaptic marker Synapsin 1 / Nuclear staining DAPI. (D) Pan-neuronal marker MAP2 / Vesicular GABA transporter VGAT/ Nuclear staining DAPI. (E-G) NeuCyte’s iNs mature very fast to form complex network, including mature synapses and spine-like structures. (E) Pan-neuronal marker TUJ1 / Nuclear staining DAPI. (F) Pan-neuronal marker MAP2 / Synaptic marker Synapsin 1. (G) Zoom in of spine-like formations on tdTomato labeled glutamatergic excitatory neuron.
SynFire iNs demonstrate principal neurophysiological properties
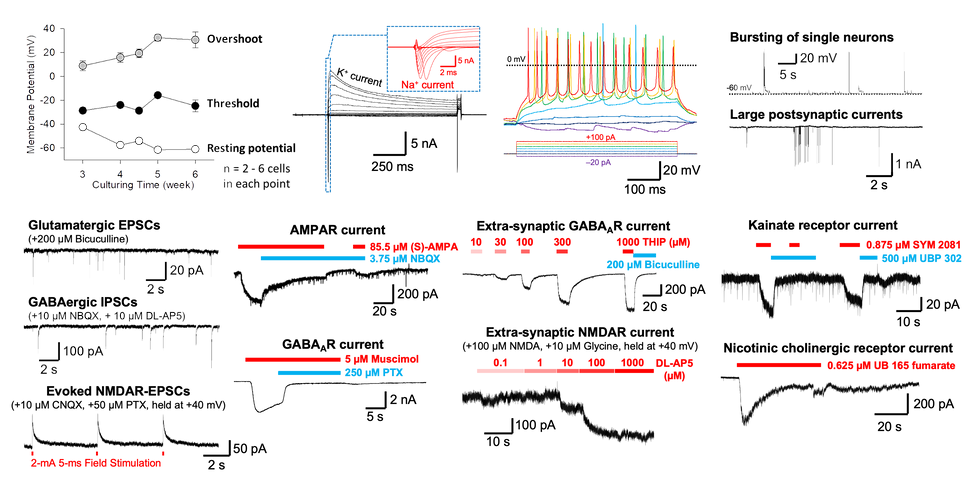
A
B
D
I
H
C
E
G
F
(A) SynFire neural cultures rapidly mature, reaching a resting membrane potential ≤ –60 mV within 5 weeks and showing stable excitability (action potential threshold and overshoot). Patch-clamp studies show intrinsic and extrinsic properties in mature SynSire neural cultures, including (B) voltage-dependent K+- and Na+-currents, (C) evoked potential action firings, (D, top) bursting of single neurons, and (D, bottom) large postsynaptic currents indicating advance synaptic competence. (E) Pure SynFire subtype cultures of either (top) only excitatory iNs or (bottom) only inhibitory iNs exclusively show glutamate-mediated excitatory post-synaptic currents (EPSCs) or GABA-mediated inhibitory post-synaptic currents (IPSCs), respectively. (F) Showing robust NMDA currents, mature SynFire neural cultures are suited for studying short- and long-term plasticity. (G–I) The function of ionic receptors expressed in SynFire iNs were determined by microperfusion of their agonists or antagonists, including (G, top) AMPA-, (G, bottom) GABAA-, (H, top) extra-synaptic GABAA-, (H, bottom) extra-synaptic NMDA-, (I, top) kainate-, and (I, bottom) nicotinic cholinergic receptors.
Rapid maturation of neural network activity observed through ontogeny

These co-cultures contain 70% Glutamatergic, 30% GABAergic neurons and human astrocytes. (A) Representative raster plots from MEA recordings at weeks 1-4. Axion 48 well MEA plates were used to assess activity. (B) Graphs quantifying multiple MEA parameters. i. number of active electrodes; ii mean firing rate; iii. ISI coefficient of variation.
SynFire co-cultures demonstrate predictive responsiveness to GABA and AMPA modulators
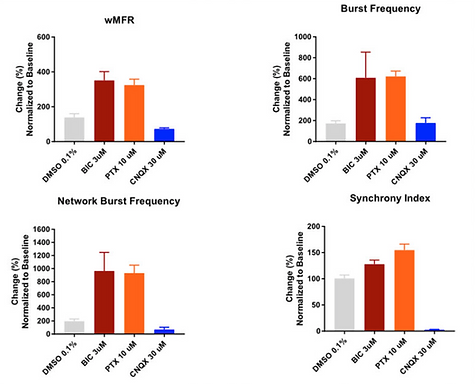
Neuronal firing and network activity were assessed in SynFire co-cultures after dosing with the GABA-A blockers Bicuculline (BIC 3 µM) and Picrotoxin (PTX 10 µM) or the AMPA blocker CNQX (30 µM). Changes in weighted mean firing rate (wFMR), burst frequency, network burst frequency and synchrony index were measured using Axions’ MEA plates. GABA blockers have an organizing effect on the network firing. Meanwhile, AMPA blockers cause a break-down in synchronous firing.
Independent comparison of SynFire neural cells to other iPSC-derived neurons demonstrates superior functionality
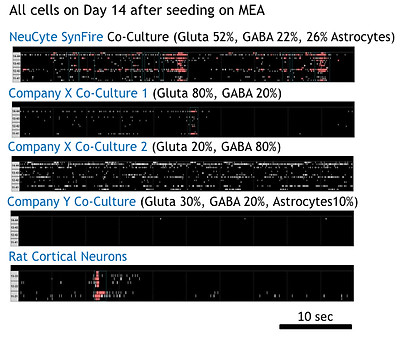
Raster plots show neural network activity of induced neural co-cultures and other commercially available neurons. Data was recorded on Axion MEA plates. Recording: Maestro/Axis, burst/network burst detector ON, default setting for spontaneous firing; rat neurons on 96 wells; others on 48 wells.

Plot shows the mean firing rate (MFR) of SynFire induced neural co-cultures and other commercially available neurons. MFR was assessed using Axion MEA plates. Axion Maestro Axis software Default setting for spontaneous neuron firing was used.

Wide range of
applications
Drug discovery and pre-clinical testing
-
Custom in vitro neural disease modeling
-
Development of neural cell based assays
-
Phenotypic and targeted drug screening
-
Neural subtype specific biochemistry
-
Target identification and validation in biologically relevant tissues
CNS safety/ Neurotoxicity
-
Cell death, apoptosis, autophagy and mitochondrial activity assays
-
Cell stress tests
-
Neural network physiology assessment (MEA)
-
Compound seizurogenic potential testing
-
Neurite outgrowth and morphology evaluations
-
Mechanism of action prediction by gene expression profiling
View the following application data of the SynFire iN co-cultures:
Neurite outgrowth assay using SynFire iN co-cultures

(A) SynFire neural cultures were treated with the actin filament disruptive toxin Latrunculin A (100 nM). Neurite length was assessed and quantified over a period of 44 hrs using a live imaging Incucyte system. (B) Representative images of the neurite traces from both excitatory and inhibitory neurons.
Seizure liability assay with compounds from the HESI NeuTox MEA seizure prediction initiative using SynFire iN co-cultures

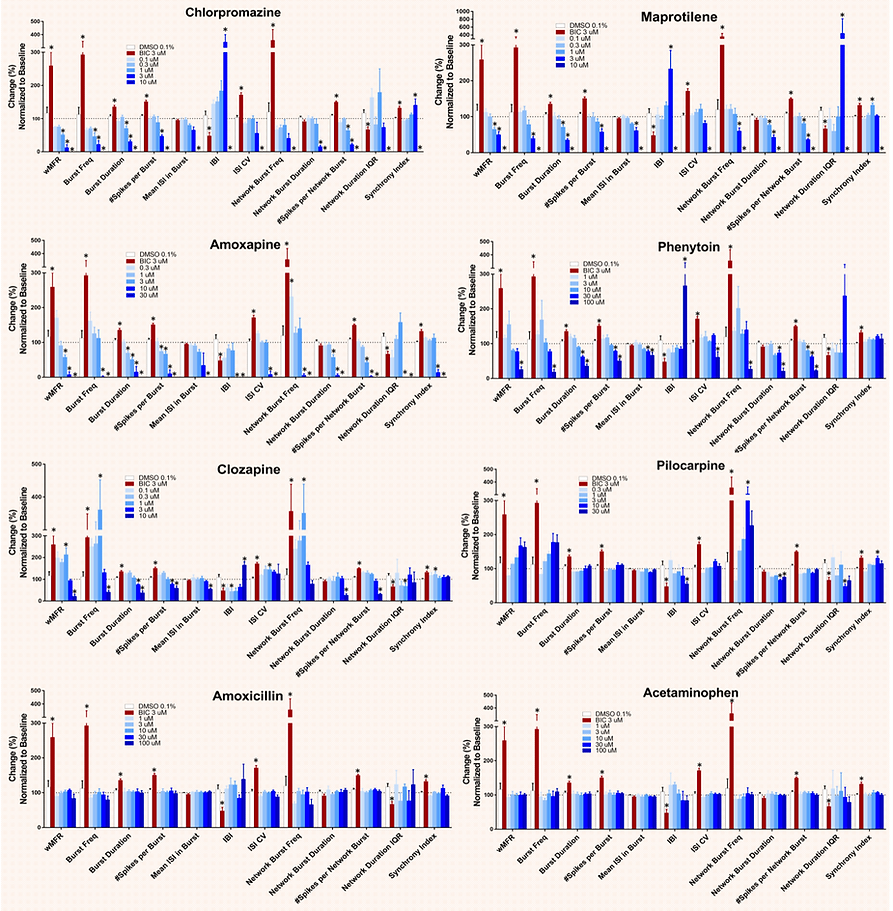
For each MEA parameter, measurements from vehicle- or compound-treated wells were normalized to their respective baseline values. All parameters are expressed as percent change. Significance for Bicuculline (positive control) relative to DMSO (Vehicle) was determined via Student's T-test (n = 4, p<0.05). Significance for test compounds relative to DMSO (Vehicle) was determined via One-Way ANOVA (n = 4, p<0.05). Pilocarpine and acetaminophen (negative control) are shown here.
SynFire neural cultures serve to test anti-epileptic drugs (AED) efficacy

NeuCyte’s iNs/MEA platform measures quantifiable effects of drugs on neuronal activity. Chemical induced seizure-like activity can be reversed in a dose dependent manner by several AEDs. Assays performed with mixed excitatory/inhibitory iN co-cultures.